Navigating the Patient Clinical Trial Training Matrix: What Path Will You Choose? (HINT: Take the Red Pill)
By Halloran Consulting Group in collaboration with Malachi Bierstein, Chief Commercial Officer at ScienceMedia, and Philip Bedrin, VP of Medical and Clinical Solutions at ScienceMedia.
Friday, Jan 19th 2024
For moviegoers, Morpheus' line, "Unfortunately, no one can be told what the Matrix is. You have to see it for yourself," followed by an offering of a red or blue pill to Neo, brings us right to the scene in the mega blockbuster hit, The Matrix.
Taking the red pill would enable Neo to understand what was occurring outside the illusion created by The Matrix, but taking a blue pill would only enable Neo to return to experiencing just that illusion.
Remember? Neo takes the red pill. Lightning flashes. Neo follows Morpheus. And we watch Neo let go of his mind's limitations, false beliefs, and attachments to those illusions, and move towards transformation.
What if we bring this offering, albeit dialed down, into the landscape of patient-facing clinical trial training? Instead of staying in the sticky illusion that the standard approach of patient training works (the blue pill), what if we examine those false beliefs, challenge our attachments to old approaches, and move towards a training process that reflects what is honest and effective?
Malachi Bierstein, Chief Commercial Officer at ScienceMedia and colleague Philip Bedrin, Vice President of Medical and Clinical Solutions at ScienceMedia, have been touting the opportunity to challenge training illusions for years to unveil that the old approach isn't working, warranting a fresh, modern take that is beneficial to clinical trial sponsors and the patients and sites they serve.
ScienceMedia executives, alongside other industry experts, gathered at CORE East, a three-day event for life science and clinical research experts produced by Halloran Consulting Group, to speak on the panel, "Trust in Innovation: Industry Leaders' Perspectives on Site and Patient Education to Drive Change in Clinical Trial Quality, Efficiency, and Engagement."
Joining the panel included Carrie Melvin, Senior Vice President, Head of Global Clinical Development Operations at Stemline Therapeutics; Colleen Graham, Vice President, Head of Clinical Operations at Mediar Therapeutics; and Tom Chen, Anti-infectives Expert at Scynexis.
To drive change and transformation, we must understand where we are. Here's a look at the current state of patient-facing training and insights gathered from the panel.
Current State of Clinical Trial Training
At most clinical trial sites, staff is given a lengthy protocol in the form of a PDF or PowerPoint document. This document is then shared with the patients at study startup (a gold standard approach relatively unchanged for over twenty years) and yet, doesn't equate to comprehension and retention.
One of the panelists noted, "I've observed sites sharing a massive PowerPoint deck, and every single procedure and criteria was included, which led to patient (and site) confusion from the beginning. The training was not engaging, and people were distracted and fatigued."
Another panelist commented, "It's like we're using yesterday's technology and continuing the same way with a 'good enough' approach."
Considering the goal for patient-facing training is to clearly communicate study expectations and develop trust with patients, all without adding additional burden to their experience, this approach continually misses the mark.
When we factor in training time, engagement, and retention, the old approaches don't provide the return on investment (ROI) for clinical trial sponsors. In addition, there are many frustrations experienced on behalf of the patient-they're overwhelmed, confused, and tired, which can lead to mistrust and dropping out of the trial.
Doesn't bode well for ROI, does it?
Transforming Patient-Facing Training for Enhanced Returns
Malachi Bierstein stated, "First and foremost, there are different modalities to share information with the sites, the Clinical Research Organizations (CROs), the sponsors, and the patients, in an engaging approach. This is the 'science of learning,' which is the ScienceMedia way to get the trial done faster, become more efficient, and deliver a better participant experience throughout the entire lifecycle of the trial."
Patients want efficient processes, micro-dosed information throughout the trial, and to hold engaging discussions about their understanding, experience, and expectations. Sponsors also want their vision and voice to come through to the patients, and a way to communicate that voice is through bite-sized, consistent, reliable patient-facing training.
Philip Bedrin noted, "Training is truly a discipline itself, and to achieve the proper training outcome, sites must invest in enhanced training processes, be equipped to tell the sponsor's story, and relate to the patient."
A panelist also shared, "Bidirectional interaction is key; for example, take inclusion and exclusion criteria for patient enrollment. We went through the criteria with our site, and then did a case study approach and shared the criteria in a case study to reinforce the information. We saw that anything that can be done in an interactive way helps with knowledge retention and follow through."
While this example is specific to sites, the principal here also applies to patient-facing training. For example, one of ScienceMedia's Phase 3 trial clients approached them with a training delivery and retention problem. In return, ScienceMedia delivered on-demand, multi-format training, and the client reached regulatory milestones and approval.
Whether it's a ScienceMedia product or a similar tool, we challenge sponsors to think differently and educate patients and sites on what they are trying to accomplish, so sites may weave that consistent messaging throughout all patient interactions.
Examples and case studies have shown there is a ROI when adopting an enhanced clinical trial training and competency tool, whether that return be in the form of faster clinical timelines, patient satisfaction, reduced patient dropout, improved core competencies and knowledge retention at the sites, being a "sponsor of choice," and sponsor satisfaction, just to name a few.
Let us visit The Matrix example one more time. If maintaining the current state of training is the blue pill, and the red pill represents taking a different path, what is the rationale to continue with the current state given the lack of return sponsors often face and the poor experiences felt by patients and sites?
Navigating The Matrix
Discovering challenges and failures within clinical trial training is common. As you observe those challenges unique to your trials, you'll be at a critical point; do you continue on the same path or take a different road?
If you are navigating your choice, here are potential results that you could be walking away from.
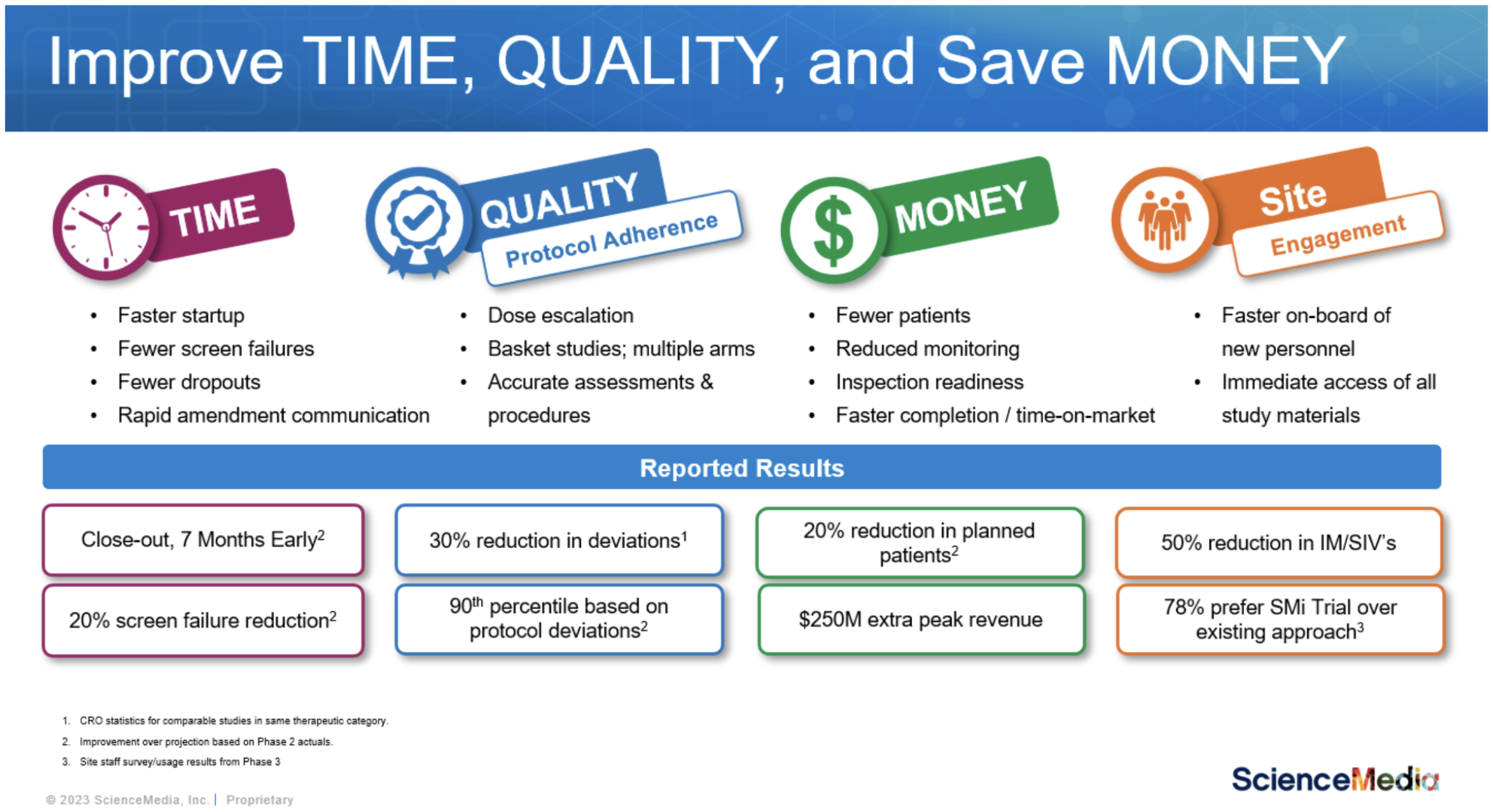
We also acknowledge change is not easy and moving from the old approach to a new way of operating requires building your case, sharing anticipated ROI with decision makers at your organization, bringing those leaders along with you, and qualifying and selecting a vendor.
We have seen the power of an engaging, multi-format training approach. The choice is yours; continue with the stale, ineffective approach or step into a new direction. Perhaps, at that point, you'll see the value for yourself and for what it really is.
About ScienceMedia
ScienceMedia empowers biopharma, medtech and CROs to embrace the science of learning and digital innovation to shorten trial timelines up to 20%, quicken site activations and save R&D spend, and propel therapeutic area training.
Our SMi TrialTM protocol learning technology eradicates protocol deviations and screening failures while reducing participant drop-outs and improving data quality.
With 25 years of training experience, ScienceMedia pioneered a proprietary methodology aligned with cognitive and behavioral multimodal learning theories: ScienceMedia MindFlowTM. The result is mind-engaging, retainable bite-sized learnings embedded in all our products. Our SMi SourceTM cloud-based platform is a medically validated library of 360+ hours of video microlearnings and 400+ disease area and clinical science courses for learning and development across R&D, Clinical Operations, Medical Affairs, and Commercial organizations.
Partner with ScienceMedia to revolutionize trial efficiencies and therapeutic area training, fostering success for sites, diverse patients, and your teams. Follow their LinkedIn to join the revolution.
About CORE
CORE, a Clinical Operations Retreat for Executives produced by Halloran Consulting Group, was launched in 2004 on the East Coast of Massachusetts and has since expanded to the West Coast in California. CORE brings together an exclusive group of senior leaders in clinical development to discuss and debate the most pressing issues around the business of product development and building enduring companies in this space.
What to learn more about CORE? Contact us today.