SMi Source lesson Biotech: Protein Formulation Physical Aspects has the following microlearning topics
1. Protein Composition

2. Protein Structure


3. Immunogenicity


4. Chemical Process

5. Physical Processes

Lesson Biotech: Protein Formulation Physical Aspects teaches these concepts
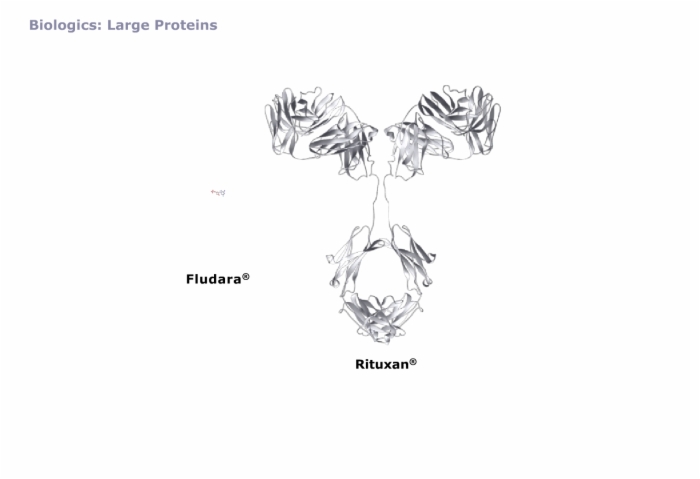
The Physical Aspects of Proteins in Formulation, Protein Composition, Biologics: Large Proteins
The Physical Aspects of Proteins in Formulation, Protein Composition, Structually Important Side Chains
The Physical Aspects of Proteins in Formulation, Protein Composition, Knowledge Check: Categorize These Amino Acids
The Physical Aspects of Proteins in Formulation, Protein Composition, Amino Acids are Polymerized to Form Protein
The Physical Aspects of Proteins in Formulation, Protein Composition, Proteins Are Made Up of Amino Acids
The Physical Aspects of Proteins in Formulation, Protein Composition, Aliphatic Side Chains
The Physical Aspects of Proteins in Formulation, Protein Composition, Aromatic Side Chains
The Physical Aspects of Proteins in Formulation, Protein Composition, Charged Side Chains
The Physical Aspects of Proteins in Formulation, Protein Composition, Amide Side Chains
The Physical Aspects of Proteins in Formulation, Protein Composition, Sulfur Containing Side Chains
The Physical Aspects of Proteins in Formulation, Protein Composition, Alcohol-Containing Side Chains
Lesson Biotech: Protein Formulation Physical Aspects addresses these key points
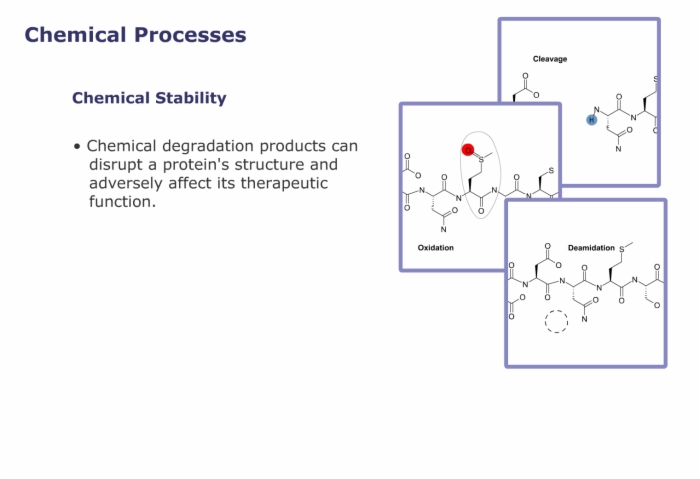
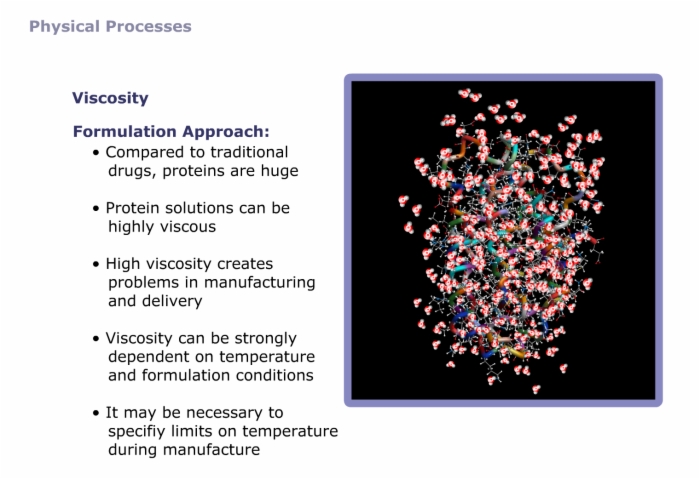
Compared to small molecule drugs, proteins are extremely large, with unique structural requirements and numerous modes of degradation.
Amino acids glycine and proline are important more for their structural than chemical properties. Glycine is the most flexible amino acid. Proline is the most constrained.
Amino acids are polymerized to form proteins by the condensation of an amine group and a carboxylic acid group to form a peptide bond.
All proteins are made from 20 naturally occurring amino acids. Each amino acid has a different side chain attached to the alpha carbon. These can be organized into groups based on the chemistry of the side chain.
Amino acids with aliphatic side chains are alanine, valine, leucine and isoleucine. They are hydrophobic and typically influence a protein’s 3D structure by occupying the interior regions.
Amino acids with aromatic side chains are tyrosine, phenylalanine and tryptophan.
- hydrophobic and often found in the protein’s interior.
- can help with stability since they absorb light in the UV region, which can lead to oxidation. Upon oxidation, tyrosine can form dityrosine which can covalently link protein molecules together.
Amino acids with carboxylic acids in the side chains are glutamic acid and aspartic acid. Amino acids with basic groups are lysine and arginine. The side chains are charged when used at the formulation pH range of 5-8.
Some histidine residues can be charged at formulation pH ranges because of its pKa. Histidine is one of the amino acids subjected to oxidation.
Amino acids glutamine and asparagines, which are amide analogs of glutamic acid and aspartic acid.
- sites for post-translational chemical modification, like glycosylation.
- important to stability and can easily be converted to glutamic or aspartic acid, known as deamidation.
Amino acids containing sulfur are methionine and cysteine.
Cysteine - important to tertiary structure since it can form cross links with other cysteines elsewhere in the protein. Both cysteine residues are susceptible to oxidation with important consequences for long term stability.
Amino acids with alcohol in their side chains are serine and threonine. The alcohol groups influence structure because of hydrogen bond capabilities with other residues and water. They are also linkage sites for glycosylation.
Lesson Biotech: Protein Formulation Physical Aspects introduces and defines these terms
Proteins: Polymers of amino acids
Amino acids: Have a common backbone component made of an amine group, a carboxylic acid, and an intervening carbon, the alpha carbon.
Deamidation